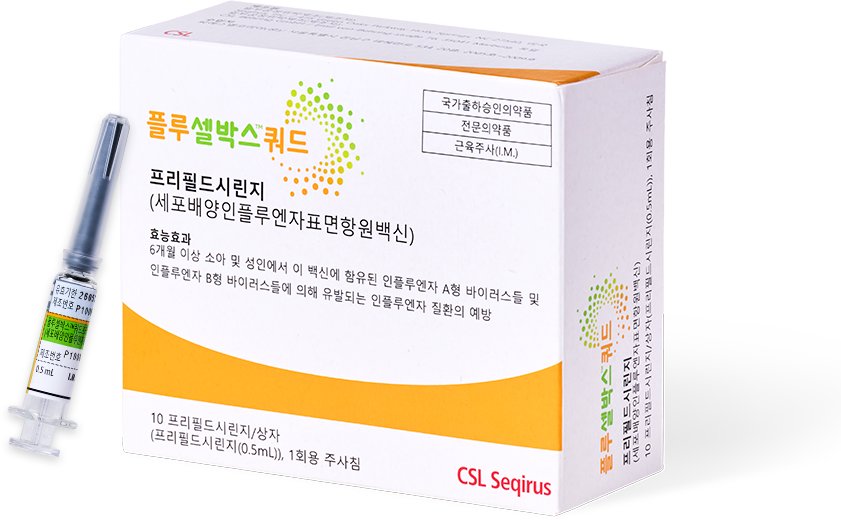
- 플루셀박스TM쿼드란?
- 접종가이드
플루셀박스TM쿼드는 어떠한 백신인가요?
6개월 이상 소아 및 성인에서 인플루엔자 질환(독감)을 예방1
세포 유래 바이러스 기반의 세포배양 독감백신2-4
유행 독감 바이러스와의
높은 항원 일치율5
다양한 실제 임상 데이터를 통해 확인된 독감 감염 예방 효과6-14
미국 FDA, 유럽EMA, 호주 등 주요국 승인(2024년 12월 31일 기준)15-18
플루셀박스TM쿼드의 접종 대상은 누구인가요?
6개월 이상 소아 및 성인
플루셀박스TM쿼드는 6개월 이상 소아 및 성인을 위한 4가 세포배양 독감백신입니다.1
플루셀박스TM쿼드연령별 접종 용량과 일정은 어떻게 되나요?
연령
용량1
투여 일정1
6개월부터 8세까지
0.5 mL씩 1회 또는 2회* 용량
2회 용량인 경우, 최소 4주 간격을 두고 접종
9세 이상
1회 용량, 0.5mL
1회 접종
플루셀박스TM쿼드의 접종 방법은 어떻게 되나요?
프리필드시린지에 부착된 주사기로 상완삼각근의 발달이 덜 된 소아의 경우 대퇴부 전외측, 그 외의 소아 및 성인의 경우 상완삼각근에 근육주사합니다.1
REFERENCES

- 식품의약품안전처 의약품통합정보시스템. 플루셀박스쿼드프리필드시린지(세포배양인플루엔자표면항원백신) https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetailCache?cacheSeq=202401917aupdateTs2025-01-25%2013:18:41.0b Accessed on July 31, 2025.
- Milián & Kamen. Current and Emerging Cell Culture Manufacturing Technologies for Influenza Vaccines. Biomed Res Int. 2015;2015:504831.
- Rajaram et al. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther Adv in Vacc and Immunoth. 2020;8:2515135520908121.
- Centers for Disease Control and Prevention. Cell-based flu vaccines. https://www.cdc. gov/flu/protect/vaccine/cell-based.htm. Accessed on July 31, 2025.
- Rajaram S, et al. Retrospective Assessment of the Antigenic Similarity of Egg-Propagated and Cell Culture-Propagated Reference Influenza Viruses as Compared with Circulating Viruses across Influenza Seasons 2002–2003 to 2017–2018. Int J Environ Res Public Health. 2020;17(15):5423.
- Klein NP, et al. Vaccine effectiveness of cell-culture relative to egg-based inactivated influenza vaccine during the 2017-18 influenza season. PLoS One. 2020;15(2):e0229279.
- Boikos C, et al. Relative Effectiveness of the Cell-Cultured Quadrivalent Influenza Vaccine Compared to Standard, Egg-derived Quadrivalent Influenza Vaccines in Preventing Influenza-like Illness in 2017–2018. Clin Infect Dis. 2020;71(10):e665-e671.
- Stein AN, et al. Relative Vaccine Effectiveness of Cell- vs Egg-Based Quadrivalent Influenza Vaccine Against Test-Confirmed Influenza Over 3 Seasons Between 2017 and 2020 in the United States. Open Forum Infect Dis. 2024;11(5):ofae175.
- Martin ET, et al. Low Influenza Vaccine Effectiveness Against A(H3N2)-Associated Hospitalizations in 2016–2017 and 2017–2018 of the Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN) J Infect Dis. 2021;223(12):2062-2071.
- Bruxvoort KJ, et al. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine. 2019;37(39):5807-5811.
- Divino V, et al. A real-world study evaluating the relative vaccine effectiveness of a cell based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season Vaccine. 2020;38(40):6334-6343.
- Divino V, et al. A Real-World Clinical and Economic Analysis of Cell-Derived Quadrivalent Influenza Vaccine Compared to Standard Egg-Derived Quadrivalent Influenza Vaccines During the 2019–2020 Influenza Season in the United States. Open Forum Infect Dis. 2021;9(1):ofab604.
- Boikos C, et al. Relative Effectiveness of the Cell-derived Inactivated Quadrivalent Influenza Vaccine Versus Egg-derived Inactivated Quadrivalent Influenza Vaccines in Preventing Influenza-related Medical Encounters During the 2018–2019 Influenza Season in the United States. Clin Infect Dis. 2021;73(3):e692-e698.
- Krishnarajah G, et al. Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–19 Influenza Season in the United States. Vaccines (Basel). 2021;9(2):80.
- FDA July 1, 2024 Approval Letter-Flucelvax Quadrivalent.
- EMA. Flucevax Tetra. Available at https://www.ema.europa.eu/en/search?f%5B0%5D=ema_search_entity_is_document%3ADocument&search_api_fulltext=Flucelvax%20Tetra Accessed on July 31, 2025.
- Australian Government Department of Health and Aged Care Therapeutic Goods Administration(TGA). Australian Public Assessment Report(AusPAR): Flucelvax Quad. Available at https://www.tga.gov.au/resources/auspar/auspar-flucelvax-quad-0 Accessed on July 31, 2025.
- GOV.UK>Health and social care>Public health>Health protection>Immunisation>Flu vaccination programme 2024 to 2025: healthcare practitioners. Available at https://www.gov.uk/government/publications/flu-vaccination-programme-information-for-healthcare-practitioners Accessed on July 31, 2025.
용어

- EMA, European Medicines Agency;
- FDA, Food and Drug Administration;
- MHRA, Medicines and Healthcare products Regulatory Agency;
- TGA, Therapeutic Goods Administration;
- WHO, World Health Organization.
광고심의필 2025-1775-106900
광고심의필 2025-1777-105700
KOR-AQIV-25-0024